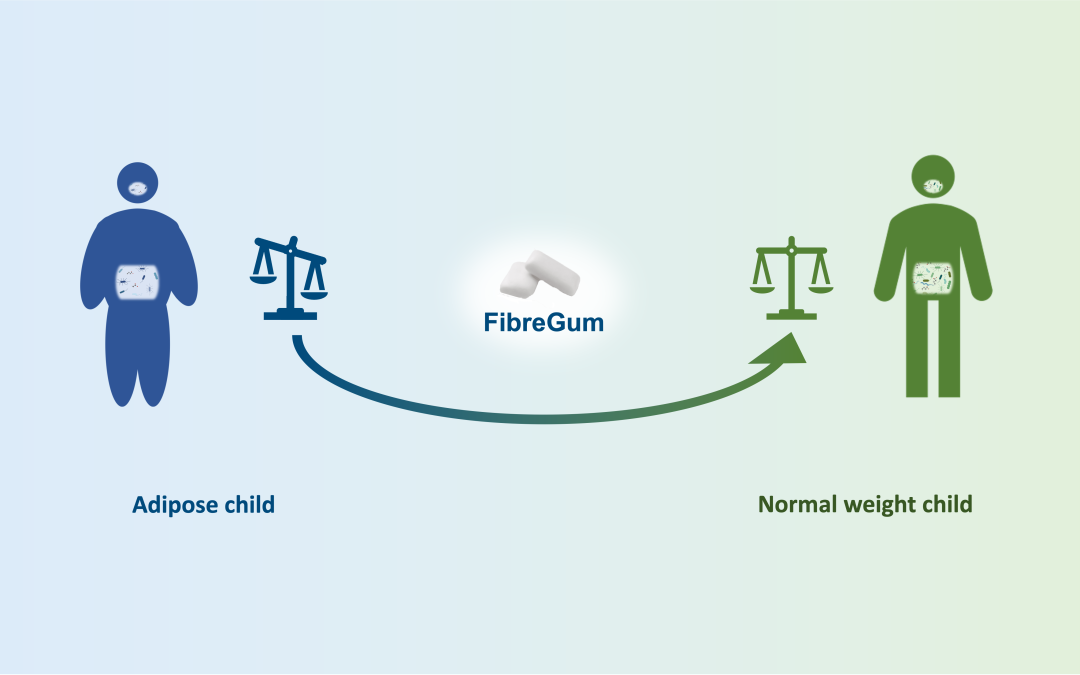
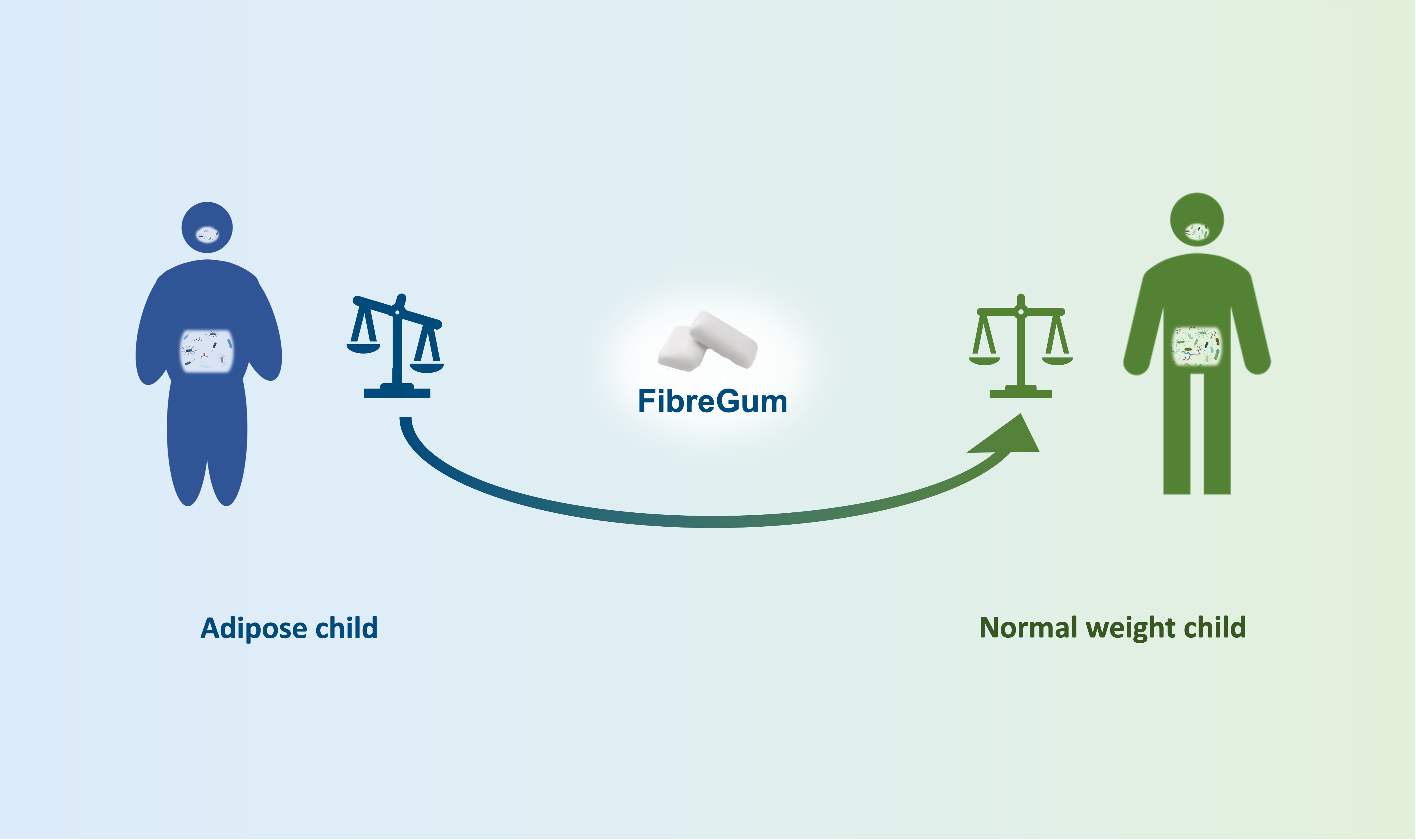
DCB and Tidepool are entering a partnership to explore the relationship between diabetes and women’s health. The first...

Menstrual Cycle Study: Identifying changes in insulin sensitivity across the menstrual cycle in T1D
Menstrual Cycle Study: Identifying changes in insulin sensitivity across the menstrual cycle in T1D
DCB and Tidepool are entering a partnership to explore the relationship between diabetes and women’s health. The first initiative in this collaboration focuses on the menstrual cycle’s influence on insulin-dependent diabetes.

Menstrual Cycle Study
Recognising the challenges faced by women with insulin-dependent diabetes, research specifically investigating the menstrual cycle’s impact on diabetes management remains limited so far. This lack of focused study has hindered the development of tailored treatment strategies for women.
Diabetes Center Berne (DCB) and Tidepool aim to fill this critical research gap. The joint effort is focused on examining the interplay between the menstrual cycle and diabetes management, with the goal of enhancing care and improving quality of life.
With the goal of supporting the development of clinical guidance, tools, and products to reduce the burden of diabetes management throughout the menstrual cycle, Tidepool and DCB are joining forces to explore the relationship between menstrual cycles and insulin-dependent diabetes. While Tidepool facilitates access to the data of people living with type 1 diabetes who menstruate and provides their expertise in data collection for this initiative, DCB provides expertise in clinical research and research infrastructure.
Project team: Dr. Martina Rothenbühler (Project Leader Data Sciences, DCB), Stefanie Hossmann (Clinical Research Scientist, DCB), Maya Friedman (Founder of the Tidepool Period Project, Tidepool), Saira Khan-Gallo (Access & Equity Lead, Tidepool)
Project partner: Tidepool
About the Study Partners

DCB supports ideas and projects in the field of diabetes technology worldwide by providing expertise, access to clinical research facilities and its own laboratories, as well as financial resources. The goal is to bring them a big step closer to market entry in a collaborative partnership. The work of the DCB is non-profit – the goals are new insights and innovations around diabetes management as well as a vibrant community.
Tidepool is a 501(c)(3) nonprofit organization. It was founded by people with diabetes, caregivers, and leading healthcare providers committed to helping all people with insulin-requiring diabetes safely achieve great outcomes through more accessible, actionable, and meaningful diabetes data.
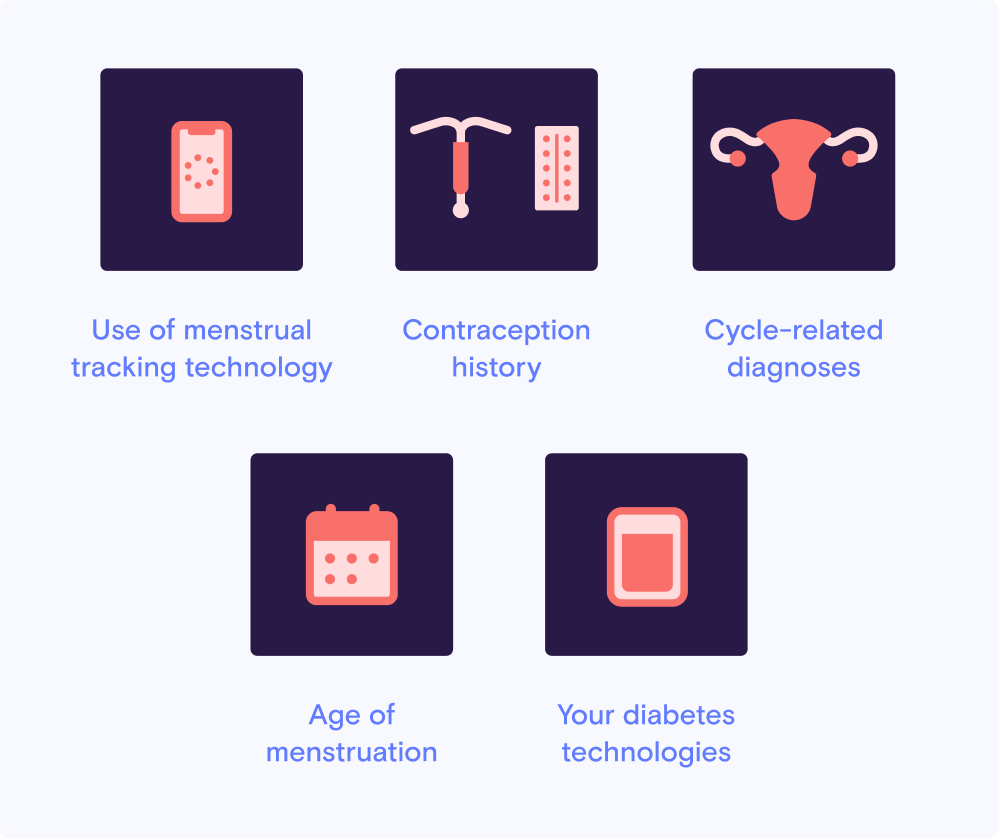
Study requirements for U.S. residents
Links
Enroll in this cycle study!
(U.S. residents only)
Contact
Menstrual Cycle Study Team
More recent projects
Menstrual Cycle Study: Identifying changes in insulin sensitivity across the menstrual cycle in T1D
enhance-d: Enhanced Diabetes Self-Management
Diabetes technology generates a lot of data, but rarely is all this data used to inform action. DCB supports enhance-d...
Qarbs: Accurate results for estimating carbohydrates
Estimating carbohydrates as supreme discipline for people living with diabetes is still depending on gut feelings and...


DCB Research AG
Freiburgstrasse 3
3010 Bern
Switzerland