Information
about the TIMES study
Read in other languages:
The TIMES study is being conducted by the Diabetes Center Berne (DCB) and aims to gain a better understanding of type 1 diabetes (T1D) throughout the menstrual cycle.
This could ultimately improve the quality of life for women with T1D.
If you are interested in participating, find more information on the study:
About us as DCB
The TIMES study is being conducted by the Diabetes Center Berne (DCB). The DCB is a non-profit foundation based in Bern, Switzerland, whose goal is to improve the lives of people with diabetes.
Aim of the study
The aim of this study is to gain a better understanding of how insulin sensitivity changes over the course of the menstrual cycle in women with type 1 diabetes (T1D). We are trying to identify patterns in how insulin requirements change over the course of the cycle, with the hope of promoting more personalized diabetes care.
This could ultimately help women with T1D achieve better health outcomes and improve their quality of life.
Background
Premenopausal women with type 1 diabetes (T1D) often experience changes in blood sugar control related to their menstrual cycle. These natural hormone fluctuations can make diabetes management difficult, especially since current AID systems are not designed to adjust insulin delivery in response to these monthly hormonal changes.
The TIMES study was developed to gain robust scientific insights into how insulin requirements and blood glucose control change during the menstrual cycle. The multinational study is being conducted in a decentralized manner and includes a total of 350 women, approximately 50 each in Switzerland, Denmark, the UK, the US, and Germany. As this is a decentralized study, all study-related activities take place online or at the participants’ homes. It is not necessary to visit a clinic or medical center.
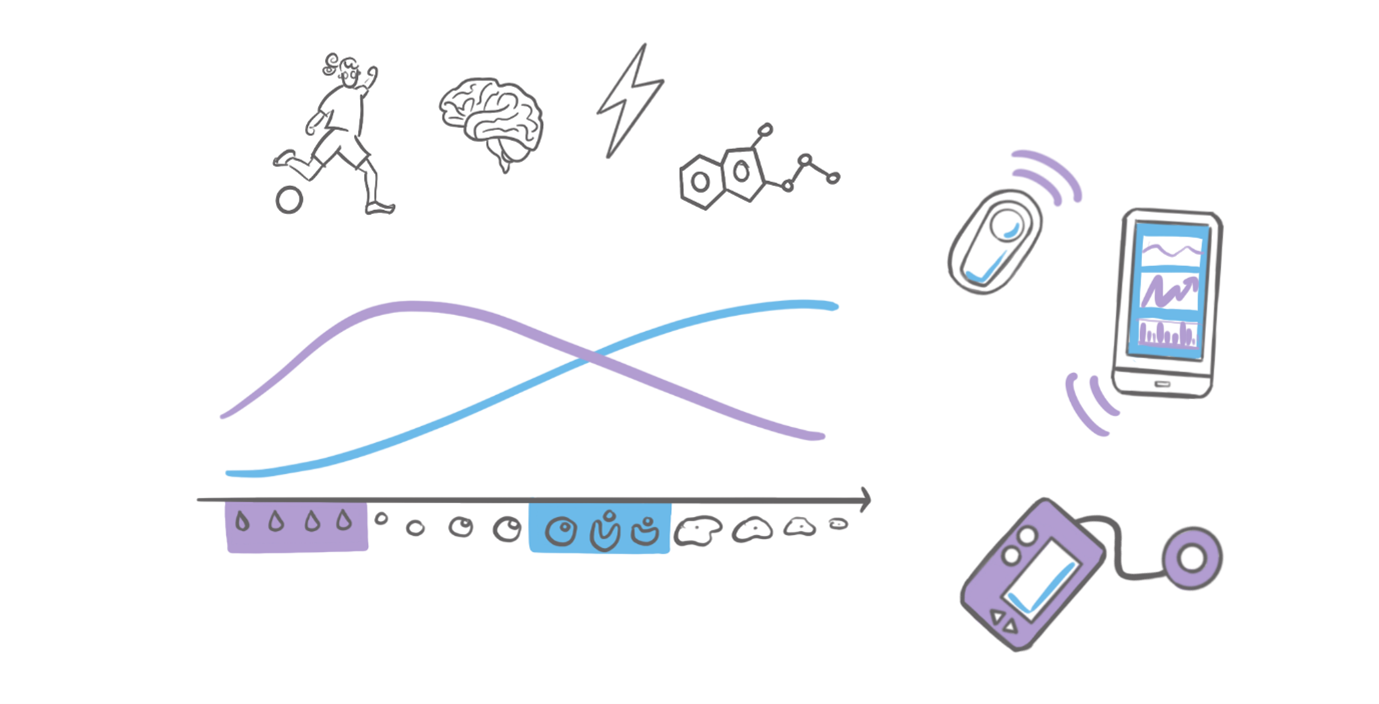
By collecting real-world data (data from everyday life) from participants’ automated insulin delivery systems (AID systems), we can obtain much of the information needed to understand how insulin requirements and blood sugar levels fluctuate during the menstrual cycle. This is supplemented by data that participants themselves enter in surveys, such as information on menstrual cycles, symptoms and lifestyle habits.
Our goal is to identify typical patterns in insulin requirements and blood glucose levels, including time in range, hypo- and hyperglycaemia, during the different phases of the menstrual cycle. We also want to understand which factors, such as premenstrual symptoms or the level of physical activity, significantly influence or characterise these fluctuations.
We are conducting the study in accordance with Swiss law. We also comply with all internationally recognised guidelines. The responsible ethics committee has reviewed and approved the research project.
What exactly does participation in a study involve?
This study is conducted exclusively from home, meaning that all study-related activities can be completed at home. We ask participants to collect data on their diabetes management and menstrual cycle over six menstrual cycles (participation can therefore last up to seven months). You will be asked to complete several questionnaires. You will also be asked to provide or upload data from your AID system, including information on glucose levels, insulin doses and carbohydrate intake. We will provide you with a Garmin smartwatch, which you should wear throughout the study (including at night), and you will need to perform ovulation tests to determine your ovulation in each cycle (up to 10 ovulation tests per month).
More details about the study processes can be found in this study information.
What are the risks and inconveniences associated with participating in a study?
There are only minimal risks associated with this study:
- Data protection and data security: Although we use secure systems, there is a small risk of data breaches. However, all data is encrypted and stored securely to protect your privacy as much as possible.
- Time and effort: Participation requires regular uploading of data and completion of questionnaires, which may be perceived as inconvenient or cumbersome.
What about my confidentiality and data protection?
All data protection requirements are strictly adhered to. The study data is stored securely in REDCap® in Switzerland in compliance with the Swiss Data Protection Act (revDSG). The data available in the Clue app and Garmin app is also stored in the European Union under the General Data Protection Regulation (GDPR). These laws are very similar.
It is possible that your data may need to be transmitted in encrypted form, for example for publication, and made available to other researchers.
Will I receive compensation for my participation?
You will receive £50 per cycle for which you provide high-quality data. In total, you can receive up to £300.
This remuneration will be transferred to you at the end of your participation in the study and depends on the completeness of your data. As data quality is crucial to the success of this study, we ask that you follow the instructions as closely as possible.
If we find that your data is incomplete, we will provide you with feedback. We are pleased to recognise all your efforts that contribute to the success of this study. The results of this research project may contribute to the development of new commercial products. Your participation does not entitle you to any claims on commercial developments (e.g. patents).
Frequently asked questions
Why was I told that I am not eligible to participate?
We greatly appreciate your interest. Unfortunately, either your initial information did not meet the study’s eligibility requirements, or the information collected during your initial data entry indicated that you no longer meet the requirements. If you wish, you can send us an email at times@dcberne.com and we will be happy to explain further.
I am interested in the TIMES study – what should I do next?
If you have read about the study and are interested, simply follow this link to continue.
Under this link you will find the participant information sheet, which contains further information about the TIMES study.
If you have any questions or comments, please send us an email: times@dcberne.com. We are here to provide you with all the information you need.
How can I download my data from my AID system?
Here you can find the instructions for exporting data from:
If your system is not listed, please contact the TIMES Study Team for assistance!
Clue App – How it works and what to do
In the TIMES study, the Clue app helps you track your cycle and symptoms. Please follow the instructions to create your account and start tracking.
Garmin: How to install the tracker?
In the TIMES study, we also ask you to wear the Garmin watch to track yourphysical activity. Please follow the step-by-step instruction for setting up yourGarmin device.
However, you may also continue using any other activity tracker you currentlyuse (with the specified exception of Apple Watches).
Supported by








